Syntheses of linear long-chain carbon (LLCC) have been underway for some time. In 2010, the Tykwinski group reported systematic chemical syntheses of polyynes up to 44 contiguous acetylenic carbons. The chains were end-capped with bulky groups (tris(3,5-di-tert-butylphenyl) [1] or tri-isopropylsilyl [2]). There are speculations that physical chain-chain separation enforced by the end-caps may stabilize the materials against cross-linking. 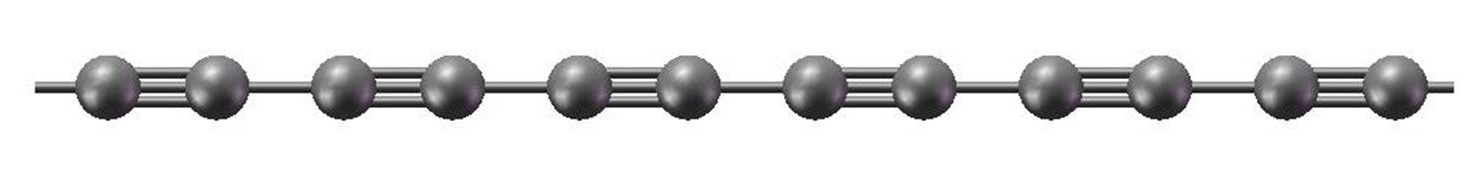
By contrast, the cumulenes have been much harder to prepare, success following only up to 9 contiguous double bonds. [3] Cumulenes are apparently delicate molecules. 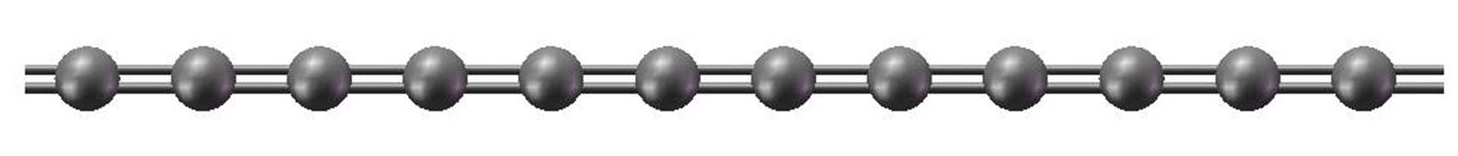
The molecular orbital (equiv. band) structures of the polyynes and cumulenes differ strongly, with the former having a distinct band gap, while the latter are metallic. However, the electronic properties are strongly influenced by the nature of the end-groups, and very likely by mechanical tension. [4,5] In bulk semiconductors, the equivalent phenomena are know as doping and strain.
A new synthetic route has been opened recently by the Pichler group. It results in LLCC embedded in the hollows of carbon nanotubes. [6] The group claims to achieve up to ~10000 carbons in the chains. So far, there is little more than Raman spectroscopy to measure the extent of the interaction between the LLCC and its protective nanotube.
Unfortunately, there are as yet few (or no) clean experimental measurements of either the electronic or mechanical properties, since isolated, uperturbed LLCC’s are not available. Nevertheless, in reading the recent literature, one gets the distinct impression that some sort of barrier has been surmounted regarding LLCC synthesis.
In addition to the Januszweksi review [3], I recommend also Milani et al. [7] concerning Raman spectroscopy of LLCCs, and Banhart [8] concerning recent LLCC developments in general.
References:
[1] W A Chalifoux, R R Tykwinski; Nature Chemistry 2, 967–971 (2010) [doi:10.1038/nchem.828]
[2] S Eisler, et al.; J. Am. Chem. Soc., 2005, 127 (8), pp 2666–2676 [doi:10.1021/ja044526l]
[3] J A Januszewski, R R Tykwinski; Chem. Soc. Rev., 2014, 43, 3184 [doi:10.1039/c4cs00022f]
[4] V I Artyukhov, M Liu, B I Yakobson; Nano Lett., 2014, 14 (8), pp 4224–4229 [doi:10.1021/nl5017317]
[5] M Liu, et al.; ACS Nano, 2013, 7 (11), pp 10075-10082 [doi:10.1021/nn404177r equiv. arXiv:1308.2258]
[6] L Shi, et al.; arXiv:1507.04896
[7] A Milani, et al.; Beilstein J. Nanotechnol. 2015, 6, 480–491 [doi:10.3762/bjnano.6.49]
[8] F Banhart; Beilstein J. Nanotechnol. 2015, 6, 559–569 [doi:10.3762/bjnano.6.58]